Topics
ai akra team article10a capa compliance device device safety eu mdr eudamed eumdr ghtf ghwp global regulations imdrf iso 14971 ivd ivdr lifecycle management mdsap medical devices medical literature review medtech orphan device paho pmcf pms post-market surveillance qms quality management system real-world data regulatory affairs regulatory harmonization risk risk management samd vigilance webinar q+a whoCAPA Fundamentals: From Compliance to Prevention

EU MDR & IVDR Insider
September 2025 Issue
A free publication from AKRA TEAM
UPDATE
Welcome to the September Issue of EU MDR & IVDR Insider
Update by Bassil Akra, PhD
INSIGHTS
- CAPA Fundamentals: From Compliance to Prevention
with Anna Spehl — AKRA TEAM
- 9 CAPA Essentials Reviewers Expect
with Azam Khorshidi — DEKRA
- How to Make CAPA the Spine of Your QMS
with Zaher Kharboutly — DNV
CAPA Metrics That Drive Action
The Best CAPA is the One You Never Have to Open
Practical CAPA Tools & Next Steps
Your CAPA Playbook

September in Review
With Bassil Akra, PhD — CEO, AKRA TEAM
EU MedTech Signals You Can Act On Now
What’s happening
After an August pause, the regulatory wheels are turning again.
September brings momentum: the Commission has opened a new MDR/IVDR consultation, fresh AI guidance is on the table, and MDSAP capacity has expanded. If you own RA/QA outcomes, these developments will shape your next quarter.
News from the EU Commission
Targeted evaluation: “Have Your Say” is open.
The Commission is collecting input on real-world bottlenecks—capacity, market availability, proportionality.
Act now: submit concise proposals (e.g., phased evidence plans, streamlined change routes, incentives that expand NB capacity). Keep examples device-class specific and tie them to continuity of supply.
MDCG guidance update
MDCG 2025-6 (with the AI Board): integrating AI Act duties into MDR/IVDR QMS.
Key threads: bias mitigation, transparency, cybersecurity—embedded in existing processes.
Focus your updates on:
-
PMS data governance and model monitoring
-
Model change control under design controls
-
Clear user labeling and HF considerations for AI features
MDSAP update
AO applications reopened (July 1, 2025).
For global portfolios, one coordinated audit can cover major markets.
Prep checklist: align internal audit cadence to MDSAP timing; map CAPA–complaints–vigilance linkages before stage-1; confirm evidence trails across QMS modules.
Why CAPA? Why now?
Each headline above converts to CAPA work—trend signals, design updates, labeling, software controls, supplier actions. A lean, risk-anchored CAPA system lets you implement changes on schedule and show objective effectiveness.
Bottom line
Contribute to the consultation, tune your QMS for AI obligations, plan MDSAP cadence, and prime CAPA to execute.
This issue distills the webinar into practical steps you can apply this week.

CAPA Fundamentals: From Compliance to Prevention
With Anna Spehl — AKRA TEAM
Understanding the Basics
Your CAPA system is a hotbed of risk.
Data from the FDA shows that for four consecutive years, CAPA-related deficiencies have been the most frequently cited issue in 483 observations. In fact, more than one-third of all FDA 483s are related to inadequate CAPA procedures.
Anna Spehl, a Senior Expert with AKRA TEAM, broke down why so many companies are failing their audits and how to get it right. During her presentation, she highlighted that the most common mistakes happen in three stages: investigation, planning, and effectiveness checks. She explained that while many of us know the process, we often fall into the same pitfalls.
"Without a structure, issues may be either missed or misclassified".
The Problem with "Human Error"
One of the most common mistakes is conducting a superficial root cause analysis, which Anna refers to as "the heart of the CAPA process." She says that without identifying the real cause, any corrective action is just a temporary fix. She challenged the overuse of "human error," which, as she points out, is not a root cause but a symptom.
As she explains, the real problem may be unclear instructions or insufficient training.
Moving from Reactive to Proactive
Anna asked the audience a critical question: "Are we solving problems or are we just documenting them?" She argued that a true proactive approach uses CAPA as a strategic tool for continuous improvement. This means:
• Trend analysis: Using data to identify issues before they become a major non-conformance. • Integrating CAPA insights: Feeding data back into your product design to improve second-generation devices. • Cross-functional teamwork: Breaking down silos so that CAPA is not just a quality team problem, but a company-wide one.
"Root cause analysis is the heart of the CAPA process. Without identifying the real cause of a problem, any corrective action is just a temporary fix."
- Anna Spehl, AKRA TEAM
"The best CAPA is the one you never have to open because you designed the issue out before it ever occurred," Anna said. She concluded by urging manufacturers to shift their focus from measuring closure rates to tracking key indicators like recurrence of causes.

9 CAPA Essentials Reviewers Expect
With Azam Khorshidi — DEKRA
From "Proactive CAPA Systems: From Compliance to Prevention," July 24, 2025
When reviewers open a CAPA file, the first pass informs them whether the record is cohesive. In this session, DEKRA Technical File Reviewer Azam Khorshidi walked through the elements that consistently earn confidence: data at the top, risk-aware decision-making throughout, and evidence that the action was effective.
1) Start with a crisp problem statement
Files that land well begin with a single sentence that names the issue, specifies where and when it appeared, and identifies the evidence source. "The procedures do not require a documented problem statement... you often realize that you don't understand the statement," she said.
2) Show the escalation rule
Reviewers look for the written criterion that moved the case from correction to CAPA—frequency, severity, trend behavior, or a defined mix. "You have to have a set criteria... through a risk based approach," she said.
3) Reach the mechanism behind "human error"
Khorshidi expected analysis that named the enabling cause—procedure clarity, interface design, training, workflow, or control-plan gaps. "Human error are rarely the reason for the quality issues," she said.
4) Match the RCA tool to the question
Five Whys works for a linear chain; Fishbone helps when multiple categories interact; Pareto helps focus. "As soon as you hit a cause that is actionable and within your control, then you're good," she said.
5) Anchor timeframes to risk and complexity
Contain quickly, then set windows that reflect design, labeling, or process change effort. "It has to be based on the risk and complexity of the issue that happens," she said.
6) Weave risk through the entire record
Strong files demonstrate how decisions were risk-informed and how outcomes informed risk assessments and benefit–risk conclusions. "You have to go back and evaluate if that risk has been identified before... and make sure that your conclusion about the benefit risk of your device is still valid," she said.
7) Keep one story across CAPA, PMS, and complaints
Auditors read interrelated processes together, especially under MDSAP. "In the MDSAP everything will be taken a look at the same time... the auditor will keep that in mind when reviewing other quality data," she said.
"Human error are rarely the reason for the quality issues."
— Azam Khorshidi, DEKRA
8) Resource it like a project
Rushed work shows. Khorshidi pointed to time, personnel, and expertise as frequent constraints and underscored multidisciplinary ownership. "Inadequate resources... that's why many of the CAPA investigations are rushed or incomplete," she said; "It should be a teamwork... it is not... the problem of the quality team only."
9) Prove effectiveness with objective criteria
Close when the outcome matches the measure tied to the changed mechanism. "You have to have something that is objective and measurable... criteria set in your plan."

How to Make CAPA the Spine of Your QMS
With Zaher Kharboutly — DNV
From "Proactive CAPA Systems: From Compliance to Prevention," July 24, 2025
A CAPA that actually moves an organization is scheduled, resourced, and easy for others to follow. In plain language, Zaher Kharboutly, Global Certification Manager for Medical Devices at DNV, described how teams keep momentum between audits, write records others can use, and show risk-based reasoning all the way to closure.
"CAPA is not something that is just paperwork... It takes time and you need to be part of your daily work, daily schedule to make sure you are addressing these non-conformities," he said.
Put process owners in the room when NCs are raised
Don't let vague findings drive weak CAPAs. Kharboutly urged manufacturers to bring the process owner to the table so the exact clause and evidence are nailed down on the spot. "The process owner needs to be present... highlight from the standard... exactly where I was not in compliance."
Keep a short weekly touchpoint
According to Kharboutly, ten minutes is enough to surface blockers and keep actions moving—so you don't see the "lump" of last-minute closures before the next audit. "Even if there's no progress, at least discuss it."
Write the record for someone who wasn't there
Auditors and colleagues must be able to pick up the file and continue the work. "You are not writing notes for yourself... it needs to be traceable," he said. Use plain language; link each action to the problem statement and the risk file; show who owns what by when.
Document schedule reality—don't rush closure
When timelines slip, explain why and keep the plan live. As Azam Khorshidi noted, "It's just better... to have a documented justification why you couldn't meet that timeline rather than... rush and close the issue."
"Deal with it at the earliest... even if there's no progress, at least discuss it."
— Zaher Kharboutly, DNV
Record the risk rationale behind market actions
If you decide against a recall or advisory, show the analysis and how you monitored the field. "We expect you to do an appropriate risk analysis... why I took that decision."
Link complaints → investigation → CAPA → effectiveness
Auditors read the story across modules—especially in MDSAP. Khorshidi reminded teams that the CAPA log can't tell a different story than PMS and complaints.
Use tools that structure thinking—not shortcuts
Root-cause and risk tools (Five Whys, Fishbone, FMEA, FTA) help multidisciplinary teams converge on the enabling mechanism and the right action. Kharboutly added that notified bodies now use a rubric to tighten how NCs and responses are written and evaluated.
Treat complaints as signals; escalate when warranted
When a customer reports an issue, register it, investigate, and then decide if it triggers CAPA. "It needs to be registered... Now if it needs to be a CAPA or not, this is going to be the next action."

CAPA Metrics That Drive Action
The Best CAPA is the One You Never Have to Open
While the best CAPA is a proactive one, you must also be ready to handle them effectively. Don't just track closure rates. Instead, focus on these metrics to truly improve your system.
MEASURING THE EFFECTIVENESS OF CORRECTIVE AND PREVENTIVE ACTIONS
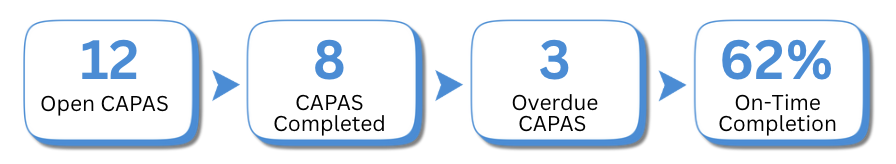
Key Metrics to Track
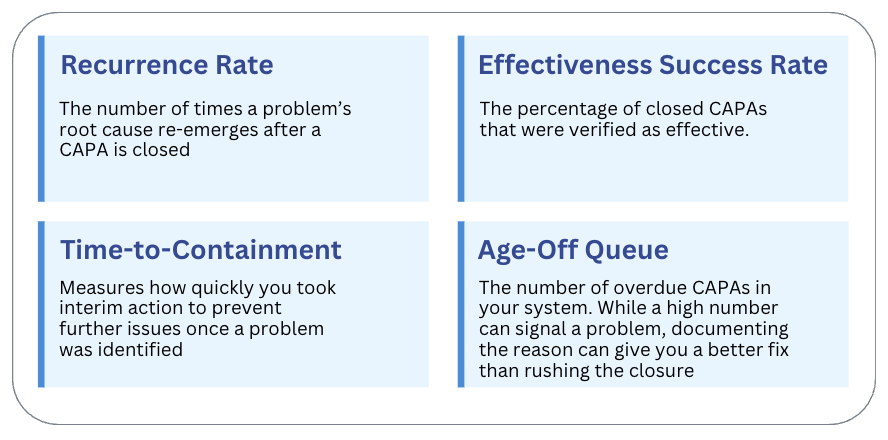
Recurrence Rate
The number of times a problem's root cause re-emerges after a CAPA is closed
Effectiveness Success Rate
The percentage of closed CAPAs that were verified as effective.
Time-to-Containment
Measures how quickly you took interim action to prevent further issues once a problem was identified
Age-Off Queue
The number of overdue CAPAs in your system. While a high number can signal a problem, documenting the reason can give you a better fix than rushing the closure
⚠️ Key Recommendations
Use a risk-based approach to make your CAPA process lean and effective. Measure recurrence rates to ensure you are actually solving problems, not just documenting them. Challenge "Human error" as a root cause, and remember that CAPA is a team sport that requires competence and sufficient training.

Practical CAPA Tools & Next Steps
A one-page playbook distilled from the AKRA TEAM webinar: take CAPA files, clear root-cause thinking, and objective effectiveness checks and apply this week
Your CAPA Playbook
Root cause tools (use, don't worship)
• 5 Whys - Linear Chains
• Fishbone - Multiple factors interact
• Bowtie - Focus where impact is biggest
Problem statement mini-template
Observed issue on device/process at location on date: found via audits/a routine complaint/field/PMS
Effectiveness, verified
Tie the checks to the changed mechanism, set objective criteria upfront, and confirm on recurrence before closing.
Meet the Experts
Anna Spehl - AKRA TEAM
QMS & Regulatory Expert for MDR/IVDR
Azam Khorshidi - DEKRA
Technical File Reviewer
Zaher Kharboutly - DNV
Global Certification Manager for Medical Devices
Keep Going
☐ Watch the replay Passcode: JeP*T1#0
☐ Download the slides (AKRA TEAM)
☐ Request a consultation on CAPA needs
About AKRA TEAM
We help MedTech and IVD manufacturers launch - and stay compliant - by aligning strategy, R&D, and rules around MDR/IVDR and global markets
EU MDR & IVDR Insider
We hope you enjoyed this issue of EU MDR&IVDR Insider, a free publication from AKRA TEAM.
Each issue is packed with expert insight, actionable analysis, and resources from top industry leaders. It's your ultimate guide to staying ahead in regulatory excellence.
September 2025 - EU MDR & IVDR Insider
© 2025 AKRA TEAM GmbH




